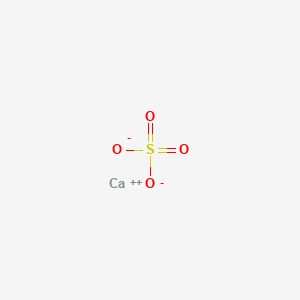
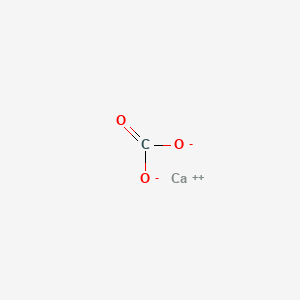
Phase At Room Temperature Of Calcium

Carbon is pronounced as kar ben.
Phase at room temperature of calcium. From the latin word for charcoal carbo. As calcite calcium carbonate it occurs on earth in limestone chalk marble dolomite eggshells pearls coral stalactites stalagmites and the shells of many marine animals. What state of matter or phase of matter at room temperature are non metals in. Calcium is a shiny metal and is solid at room temperature.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Calcium is a solid at room temperature with a density of 1 55g cm 3. Around 97 of naturally occurring calcium is in the form of the isotope 40 ca. Phase at room temperature.
Calcium is a chemical element with the symbol ca and atomic number 20. The temperature at which the liquid gas phase change occurs. What s in a name. Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
Relative atomic mass the mass of an atom relative to that of. Calcium constitutes 3 64 percent of earth s crust and 8 percent of the moon s crust and its cosmic abundance is estimated at 4 9 10 4 atoms on a scale where the abundance of silicon is 10 6 atoms. Metallic calcium was first isolated by sir humphry davy in 1808 through the electrolysis of a mixture of lime cao and mercuric oxide hgo. Although calcium is the fifth most abundant element in the earth s crust it is never found free in nature since it easily forms compounds by reacting with oxygen and water.
Two more calcium isotopes 46 ca and 48 ca have very long half lives and are considered mostly stable. Its melting point the temperature at which it turns into a liquid is 342 degrees celsius or 1548 degrees fahrenheit. Calcium has four stable isotopes including 40 ca 42 ca 43 ca and 44 ca.