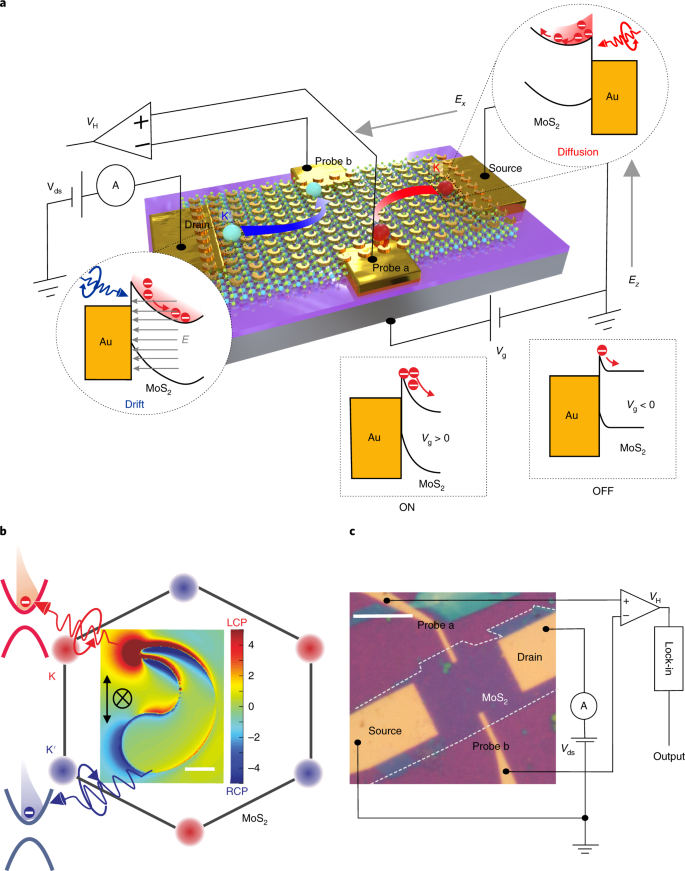
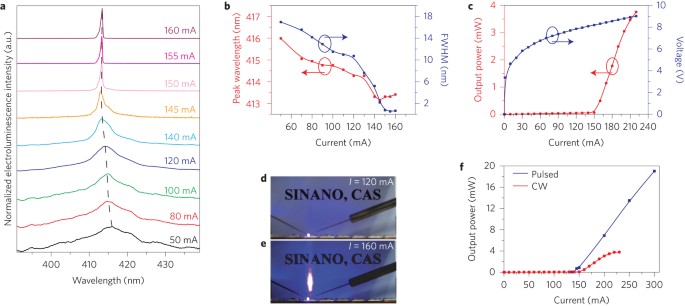
Phase At Room Temperature Of Silicon

Silicon is the eighth most abundant element in the universe and the second most abundant in the earth s crust after oxygen.
Phase at room temperature of silicon. Today silicon is produced by heating sand sio 2 with carbon to temperatures approaching 2200 c. Fluorine attacks silicon vigorously at room temperature chlorine does so at about 300 c and bromine and iodine at about 500 c. To simplify matters it is therefore assumed in the following that no carbon is soluble in the lattice of alpha iron. When high temperature 500 c nanoscratching was carried out metastable silicon phases si iii and si xii transformed into thermodynamic stable si i phase.
However so far the phase transformation. Silicon is the second element in the fourteenth column of the period table. Silicon also has promise in the creation of incredibly tiny lasers called nanoneedles which. Silicon atoms have 14 electrons and 14 protons with 4 valence electrons in the outer shell.
Aiming at exploring the non thermal annealing crystallization of amorphous silicon is carried out by femtosecond laser irradiation. In comparison with previous reports on the mispc of a si. Silicon is the seventh most abundant element in the universe and the second most abundant element in the earth s crust. Relative atomic mass the mass of an atom relative to that of.
Phase transformation from amorphous to polycrystalline confirming by the comparison of surface morphologies and micro raman spectra is demonstrated to be realized even at room temperature. This oxide layer nevertheless does not prevent reaction with the halogens. The metal induced solid phase crystallization mispc method with nickel surface coverage is used. Two allotropes of silicon exist at room temperature.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature. H the crystallization temperature is reduced by more than 350 c. Phase at room temperature. H can be instantaneously crystallized at room temperature is reported.
The temperature at which the liquid gas phase change occurs. It is classified as a member of the metalloids. Also have noted that there were si i si xii and si iii in the scratching track when nanoscratching at room temperature. Abstract a way in which thin films of hydrogenated amorphous silicon a si.
Lead has been known since ancient times. Silicon reacts with gaseous sulfur at 600 c and gaseous phosphorus at 1000 c. It is sometimes found free in nature but is usually obtained from the ores galena pbs anglesite pbso 4 cerussite pbco 3 and minum pb 3 o 4 although lead makes up only about 0 0013 of the earth s crust it is not considered to be a rare element since it is easily mined and refined.