R Plane Sapphire Crystal Structure

Sapphire is a single crystal al 2 o 3 with a hexagonal rhombohedral crystal structure.
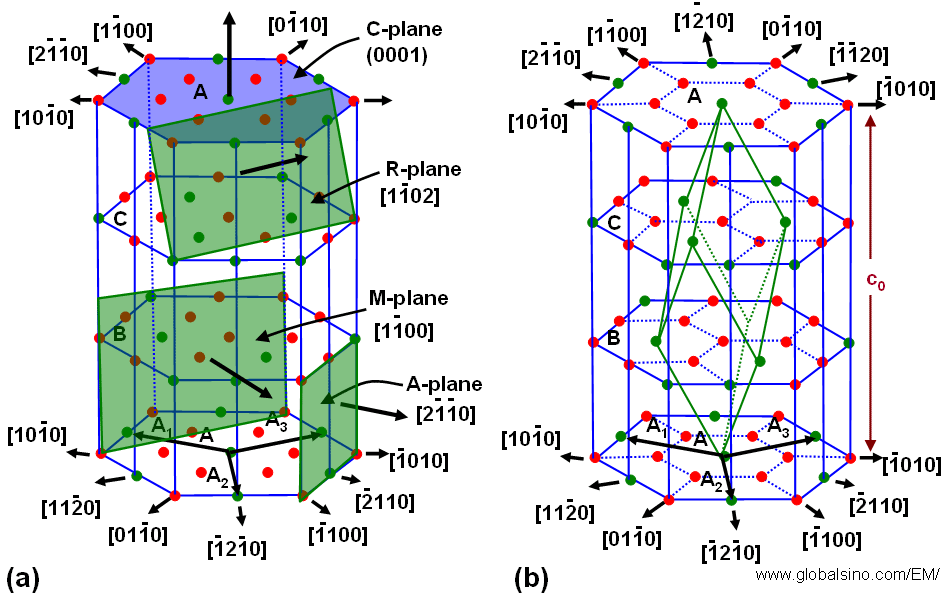
R plane sapphire crystal structure. A 0 4763 nm c 1 3003 nm standard orientation. Improved fabrication processes ensure no subsurface work damage and the exceptionally smooth surface finish and high dielectric constant can make sapphire an excellent choice for hybrid substrates particularly for microwave acs. On r plane sapphire the epitaxial formation of 110 oriented cubic fe doped in 2o 3 has been reported 21 without any information on domain structure and in plane orientation whereas co doped in 2o 3 has been reported to form. Hexagonal density 25 c.
3 97 g cm lattice constant. 1 375 å 1123 n plane. Sapphire α al 2 o 3 as a hexagonal structure belonging to the space group r3c can be expressed both as a hexagonal as well as a rhombohedral unit cell. A 4 758 å c 12 992 å crystallographic d spacing 1120 a plane.
2 379 å 1102 r plane. Physical properties of sapphire crystal structure hexagonal. In a previous paper we demonstrated the growth of zinc blende inn directly on a noncubic substrate on r plane sapphire without the use of an. The sapphire corundum structure can be represented by ordering octahedron is shown on fig 1.
Fig 2 shows the structure of the primary planes of the sapphire crystal corresponding to the structure system of sapphire. Epitaxial relationship of gaas to r plane and c plane sapphire substrates has been investigated. Sapphire substrate r plane r plane sapphire substrates are preferred for the hetero epitaxial deposition of silicon used in microelectronic ic applications. O 2 ions are in tops peaks of the octahedrons and al 3 ions are inside of octahedrons.
Sapphire is equivalent to corundum alpha al 2 o 3 and may contain only some impurities color centers which generates colors which are not reddish and then called ruby. In all cases zinc blende inn was grown on a substrate with cubic crystal structure. 0001 0 3 c plane 1102 1 r plane 1120 1 a plane 1010 1 m 100 mm 530 µm.